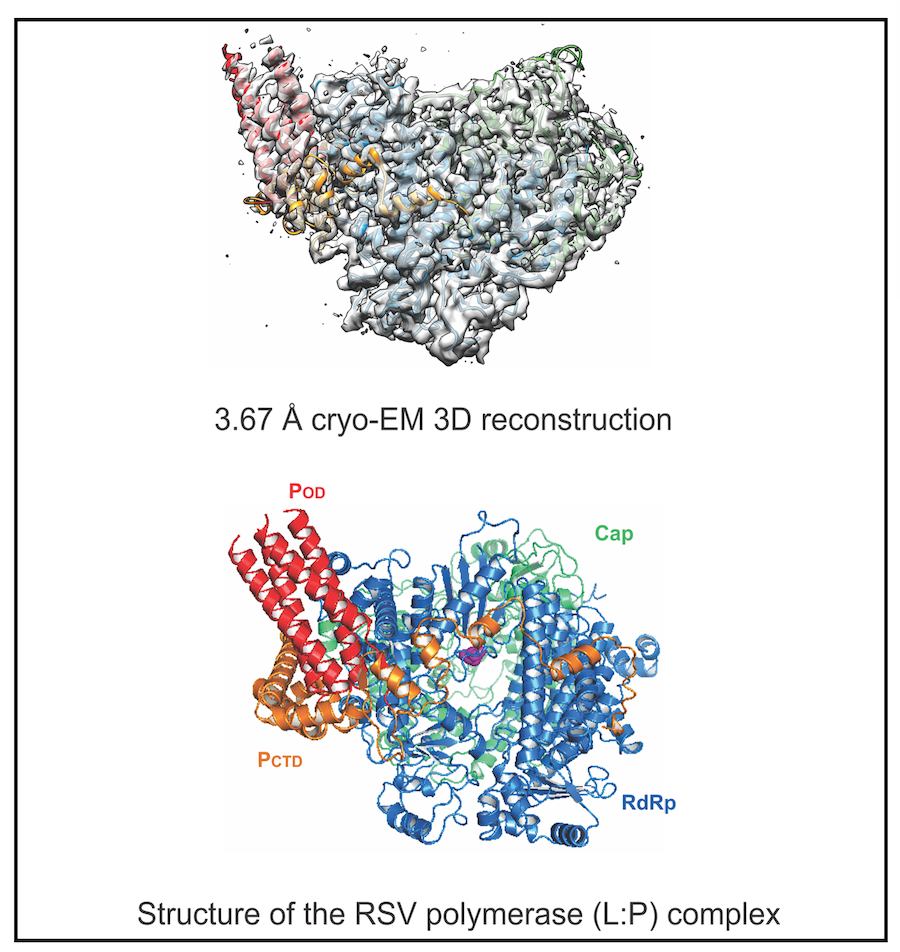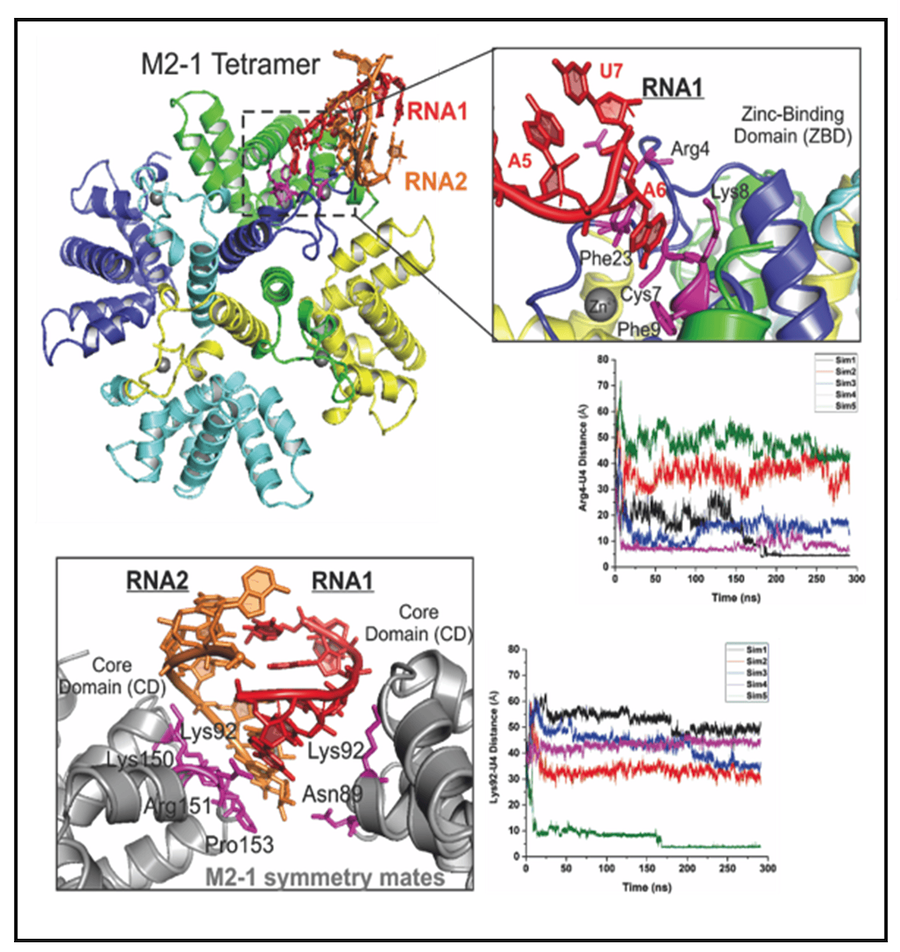
Illustration of the structure and regulation of the RSV polymerase
As the sole enzyme executing transcription and replication of RSV, the L protein is a logical target for novel antiviral drugs. However, understanding this enzyme and its cofactors at the molecular level is far from complete, impeding drug discovery efforts. The major gaps in our knowledge of the RSV RNA synthesis are the mechanistic lacking of 1) how multi-enzymatic activities of L are coordinated by its cofactor P, and 2) how L is regulated by processivity factor M2-1 during transcription to ensure that the mRNA is properly capped and polyadenylated. Importantly, robust biochemical and genetic tools are in place to dissect the enzymatic activities of L. Thus, RSV offers a clinically relevant and tractable system in which to address these knowledge gaps. We will fill these gaps by delineating the molecular biochemistry and structural enzymology of RSV L protein with its cofactor P and processivity factor M2-1. We use cryo-EM and x-ray crystallography to obtain high-resolution images at multiple vital RSV RNA synthesis stages. Furthermore, results are expected to offer new mechanistic insight into the regulation of RSV transcription by M2-1, a processivity factor that shares structural homology with Ebola VP30. These studies will be significant within the NNS field because RSV RNA synthesis shares a similar viral gene expression strategy with NNS RNA viruses, including rabies and Ebola.